Annual Summary of Disease Activity:
Disease Control Newsletter (DCN)
- DCN Home
- Annual Summary, 2022
- Annual Summary, 2021
- Annual Summary, 2020
- Annual Summary, 2019
- Annual Summary, 2018
- Annual Summary, 2017
- Annual Summary, 2016
- Annual Summary, 2015
- Annual Summary, 2014
- Annual Summary, 2013
- Annual Summary, 2012
- Annual Summary, 2011
- Annual Summary, 2010
- Annual Summary, 2009
- Annual Summary, 2008
- Annual Summary, 2007
- Annual Summary, 2006
- Annual Summary, 2005
- Annual Summary, 2004
- Annual Summary, 2003
- Annual Summary, 2002
- Annual Summary, 2001
- Annual Summary, 2000
- Annual Summary, 1999
- Annual Summary, 1998
- Annual Summary, 1997
Related Topics
Contact Info
Streptococcus pneumoniae Invasive Disease, 2012
Statewide active surveillance for invasive Streptococcus pneumoniae (pneumococcal) disease began in 2002, expanded from the metropolitan area, where active surveillance was ongoing since 1995. In 2012, 503 (9.4 per 100,000) cases of invasive pneumococcal disease were reported. By age group, annual incidence rates per 100,000 were 9.4 cases among children aged 0-4 years, 2.1 cases among children and adults aged 5-39 years, 11.9 cases among adults 40-64 years, and 28.9 cases among adults aged 65 years and older.
In 2012, pneumonia occurred most frequently (56% of infections), followed by bacteremia without another focus of infection (25%), and pneumococcal meningitis (5%). Forty-three (9%) cases died. Health histories were available for 40 of the 43 cases who died. Of these, 39 had an underlying health condition reported. The conditions most frequently reported were atherosclerotic cardiovascular disease (13), chronic obstructive pulmonary disease (10), diabetes (9), heart failure/congestive heart failure (7), and solid organ malignancy (7).
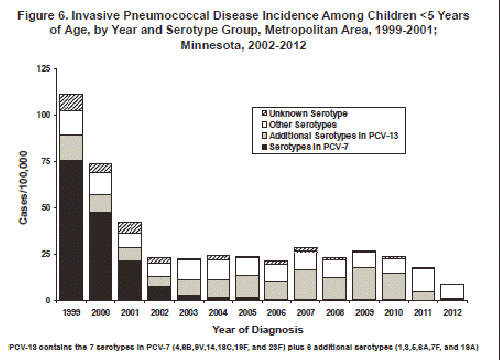
In 1999, the year before the pediatric pneumococcal conjugate vaccine (Prevnar [PCV-7]) was licensed; the rate of invasive pneumococcal disease among children <5 years of age in the metropolitan area was 111.7 cases/100,000. Over the years 2000-2002 there was a major downward trend in incidence in this age group (Figure 6). Rates in each of the subsequent 8 years were level or somewhat higher, although there has not been a continuing upward trend (Figure 6). Based on the distribution of serotypes among isolates from these cases, this increase was limited to disease caused by non-vaccine serotypes (i.e. serotypes other than the 7 included in PCV-7) (Figure 6).
In March 2010, the U.S. Food and Drug Administration approved a new 13-valent pediatric pneumococcal conjugate vaccine (PCV-13 [Prevnar 13]) which replaced PCV-7. The new vaccine provides protection against the same serotypes in PCV-7, plus 6 additional serotypes (serotypes 1, 3, 5, 6A, 7F, and 19A). From 2007 to 2010, the majority of invasive pneumococcal disease cases among children <5 years of age have been caused by the 6 new serotypes included in PCV-13 (Figure 6). Since 2011, the majority of invasive pneumococcal disease cases among children <5 years of age have been caused by serotypes not included in PCV-13 (Figure 6). In 2012, 26% of cases occurring among Minnesotans of all ages, with isolates available for testing, were caused by 3 of the new PCV-13-included serotypes: 19A (7%), 3 (10%), and 7F (8%).
Of the 478 isolates submitted for 2012 cases, 95 (20%) isolates were resistant to penicillin using meningitis breakpoints. Using non-meningitis breakpoints, 7 (1%) of 478 isolates were resistant to penicillin and 16 (3%) exhibited intermediate level resistance (Note: CLSI penicillin breakpoints changed in 2008; refer to the MDH Antibiogram on pages 26-27). Multi-drug resistance (i.e., high-level resistance to two or more antibiotic classes) was exhibited in 83 (17%) isolates.
- For up to date information see>> Streptococcus pneumoniae (Pneumococcal Disease)
- Full issue>> Annual Summary of Communicable Diseases Reported to the Minnesota Department of Health, 2012